4. Instructions for submitting Research Protocols
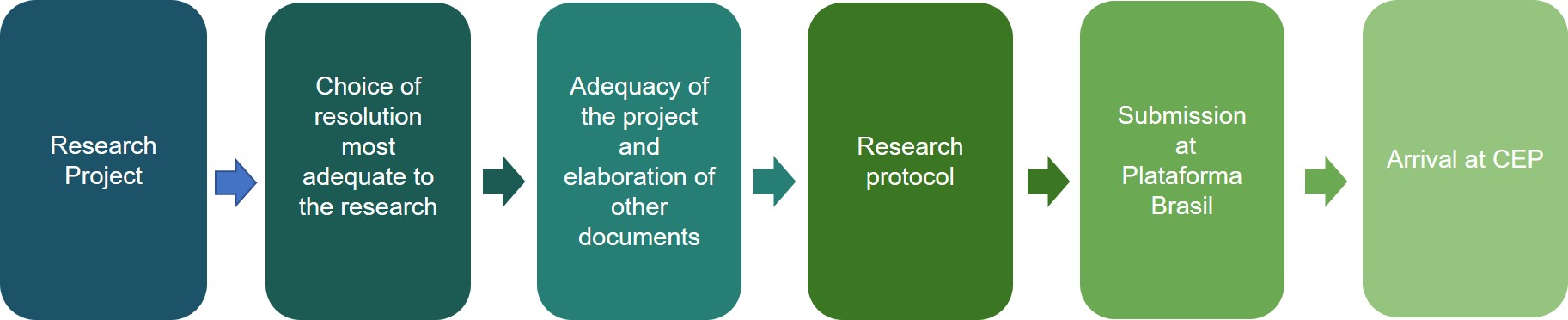
Submission of the work for the evaluation of CEP-PECEGE should follow some steps:
The researcher should do their registration as a researcher in Plataforma Brasil;
The researcher should prepare any necessary documentation that will be sent to CEP-PECEGE;
- After the organization of documents, the researcher should register their research in Plataforma Brasil, include all necessary documents and forward them to CEP-PECEGE.
It’s very important to remember that although CEP is responsible for applying ethical guidelines and evaluating research projects, it is a non transferable responsibility of the researcher to know and adopt these guidelines during the execution of the research work.
Thus, the contents presented here are intended to assist you in preparing documents and submitting them to CEP-PECEGE, however, the researcher is the main responsible for planning, organizing and executing their research and for producing and adequating the documents necessary for evaluating the ethical quality of the project.
In addition, it will be necessary to familiarize themselves with some concepts that are frequently used by the CEP/Conep System.
Next, some of the main concepts that the researcher needs to know:
Responsible researcher: if the researcher already has a complete academic or technological formation, that is, if the researcher has a higher education degree (equivalent to a Brazilian university degree), and need to submit a research project for the analysis of CEP-PECEGE, for all purposes, he is the researcher responsible for the research. According to Res. CNS 510/2016, the responsible researcher is the “person with at least the title of a bachelor*, responsible for coordinating and conducting research and for the integrity and well-being of participants in the research process. In the case of graduation students performing research for the elaboration of their Final Paper, the research will be registered in CEP, under the responsibility of the respective Final Paper advisor (item XVII, Cap. I, Res. CNS 510/2016)”.
Research protocol: in CEP/Conep, this expression is used to designate the set of documents that should be forwarded for evaluation. The research project is only one of these documents. The amount of mandatory documents will vary from research to research, depending on their complexity and their methodological design. See the menu “Protocol Organization” for more information.
Proponent institution: if the researcher is a student enrolled in PECEGE and if their research is an integral part of their graduation or post-graduation course lato sensu (specialization and MBA) or stricto sensu, PECEGE should always be indicated as the proponent institution in their documents.
4.1. Plataforma Brasil
Plataforma Brasil is the digital working platform of the CEP/Conep System It brings together researchers and institutions, CEPs and Conep itself, in a virtual environment that offers all the tools necessary to perform the evaluation and monitoring of research involving human beings in the country.
To submit the research project to CEP-PECEGE, it will be necessary to perform the registration in Plataforma Brasil.
The entire analysis process performed by CEP-PECEGE will be registered in Plataforma Brasil and the researcher may follow the progress of this process on the platform itself. In addition, all communication between the researcher and CEP-PECEGE will also be via the platform. Therefore, it is essential for the researcher to know the functionalities that Plataforma Brasil offers the researchers and use the teaching materials available to understand how this system works.
Access to Plataforma Brasil is made through the link: http://plataformabrasil.saude.gov.br/login.jsf
4.1.1. Plataforma Brasil - Researcher Registration
Researcher registration
When the researcher is using Plataforma Brasil, for the first time, it will be necessary to make a personal register. To do this registration, the researcher will need to provide a digital copy of the following documents:
- Identity document (front and verse), in PDF format.
- A photo, in JPG format.
- Summarized Lattes curriculum in PDF format.
This registration will be performed once, so that the researcher can have access to the system.
The following step by step was elaborated to assist in this process.
Alteration of the researcher registration
Plataforma Brasil is the main communication platform of the CEP/Conep System Besides exchanging files and messages through the system itself, many information are exchanged via email. Institutional connection updates are also important, because this connection determines which CEP will receive the project for analysis. Therefore, it is essential to maintain contact data and professional information updated.
The following step by step was elaborated to assist in this process.
4.1.2. Plataforma Brasil - Submission of the Research Protocol
Submission of research protocol
Whenever it is necessary to submit a research protocol to CEP-PECEGE, this submission will be done through Plataforma Brasil. The submission process is called “Submission”.
For information on how to assemble the research protocol, refer to the menu “Instructions for submission”.
It is important to know that, once the protocol has been sent to CEP-PECEGE, via Plataforma Brasil, no information can be changed until CEP manifests through a consolidated report. In other words, when completing the submission in Plataforma Brasil, the researcher is automatically requesting CEP-PECEGE to evaluate the work and no information or document may be changed from this point onwards, until the researcher receives a report from CEP-PECEGE. Therefore, be careful to verify all documentation and all information inserted in Plataforma Brasil before completing the submission.
The following step by step was elaborated to assist in this process.
Step by step for Research Protocol Submission
Editing the Submission of Research Protocol – during filling and before submitting to CEP
Since the submission process involves filling out many information and preparing several documents, it can be done in stages. The researcher can fill out work information in steps. Thus, it is possible to fill out some information, save them in Plataforma Brasil and resume the filling of the forms later.
The following step by step was elaborated to assist in this process.
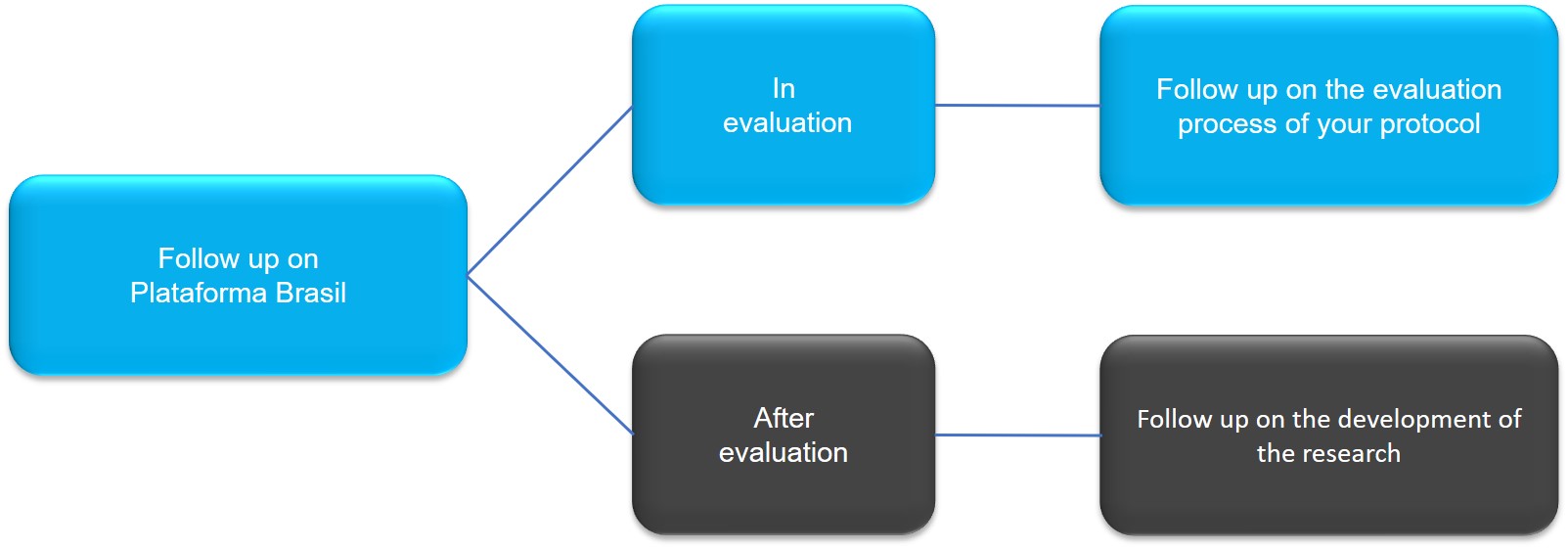
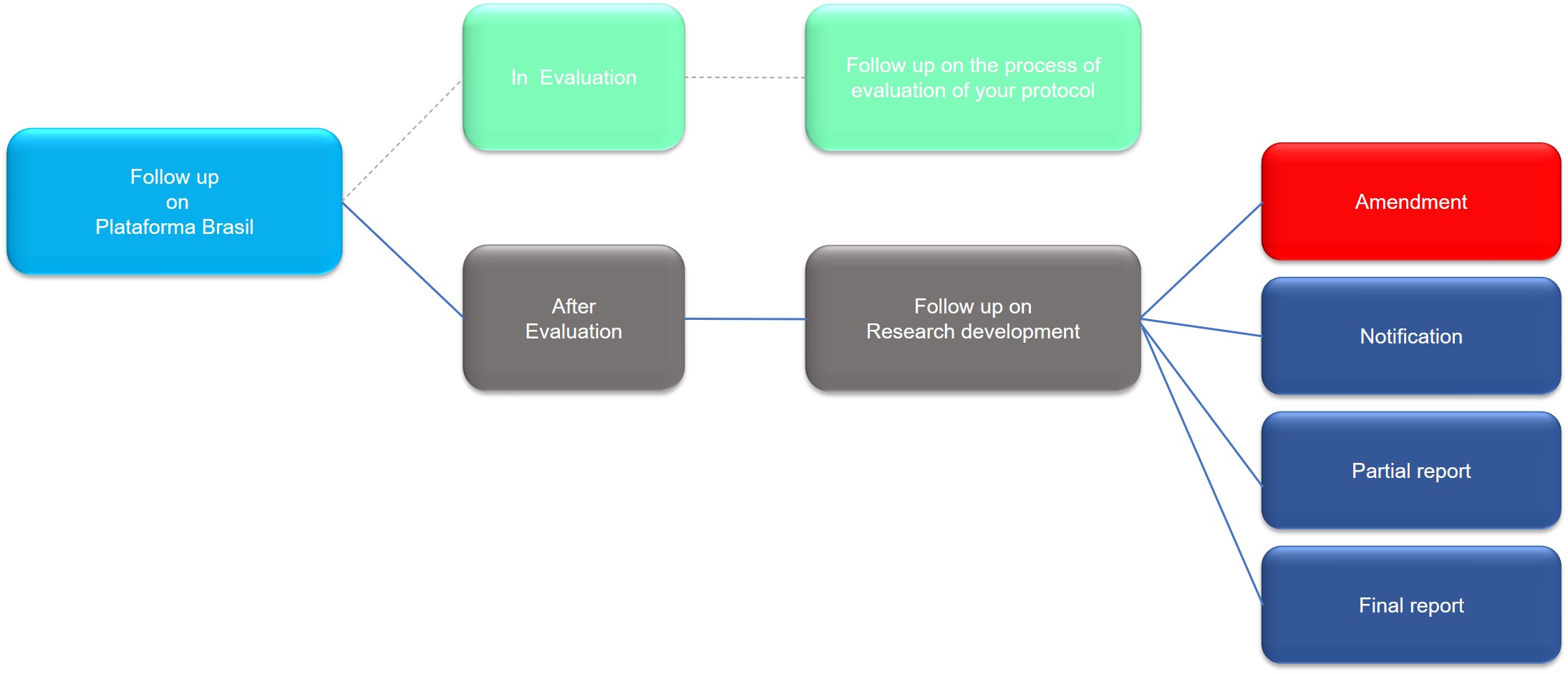
4.1.3. Plataforma Brasil - Monitoring the Research Protocol
Monitoring the research protocol in Plataforma Brasil can be divided into two moments:
- In evaluation: monitoring the evaluation process of the research protocol by CEP-PECEGE
- After evaluation: monitoring research development, after the approval of the research protocol
4.1.3.1. Plataforma Brasil – Monitoring the Research Protocol in evaluation
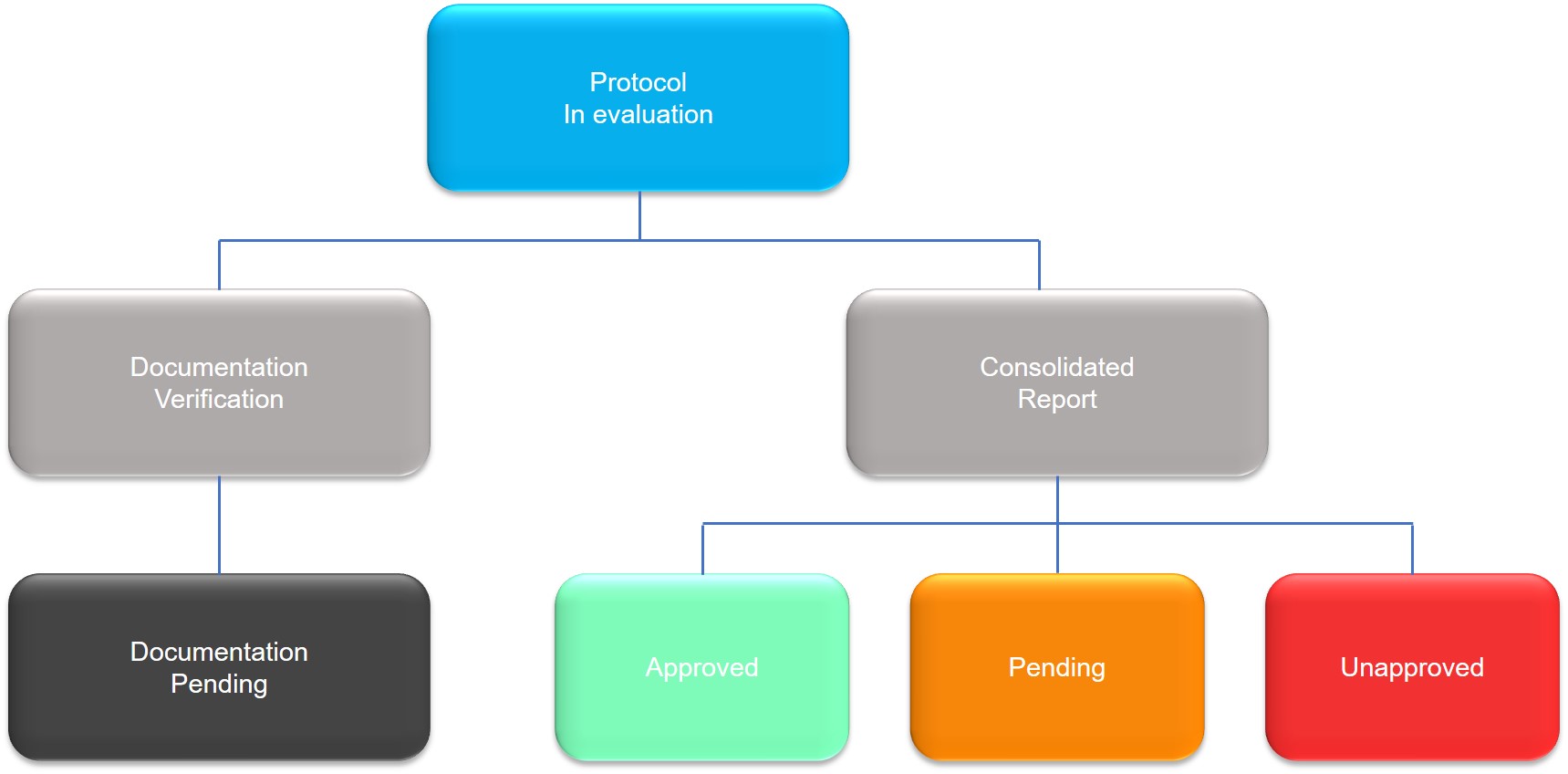
Once the research protocol is submitted to CEP-PECEGE, via Plataforma Brasil, the process of evaluating the ethical quality of the work will be initiated.
In short, CEP, after performing the evaluation, will manifest its decision in a document called Consolidated Report, in which it will report the “Protocol Status”.
The researcher will be informed by email whenever the Consolidated Report is available and may find it in Plataforma Brasil.
During the evaluation of the research protocol, four situations may arise:
- Documentation Pending;
- Approved;
- Pending;
- Unapproved.
Each of them will require from the researcher a specific response to CEP-PECEGE. To assist, the following tutorials were prepared:
1. Status of “Documentation Pending Issued by CEP”
The status of “Documentation Pending Issued by CEP” means that the protocol documentation sent to CEP is incomplete or incorrect, preventing the evaluation process from continuing.
In this case, it will be necessary to include or correct the documents indicated by CEP-PECEGE and change them directly in Plataforma Brasil.
The researcher should follow the tutorial below to respond to this situation.
2. “Approved” Project Status
“Approved” status means that the protocol was evaluated and approved by CEP-PECEGE and, therefore, the research may be initiated.
From this moment on, the researcher should check the item “Instructions for submission”, in this website, because the protocol will be monitored by CEP-PECEGE through the partial report and the final report.
In the tutorial below, the researcher will learn how to find the consolidated report of the approval of their research.
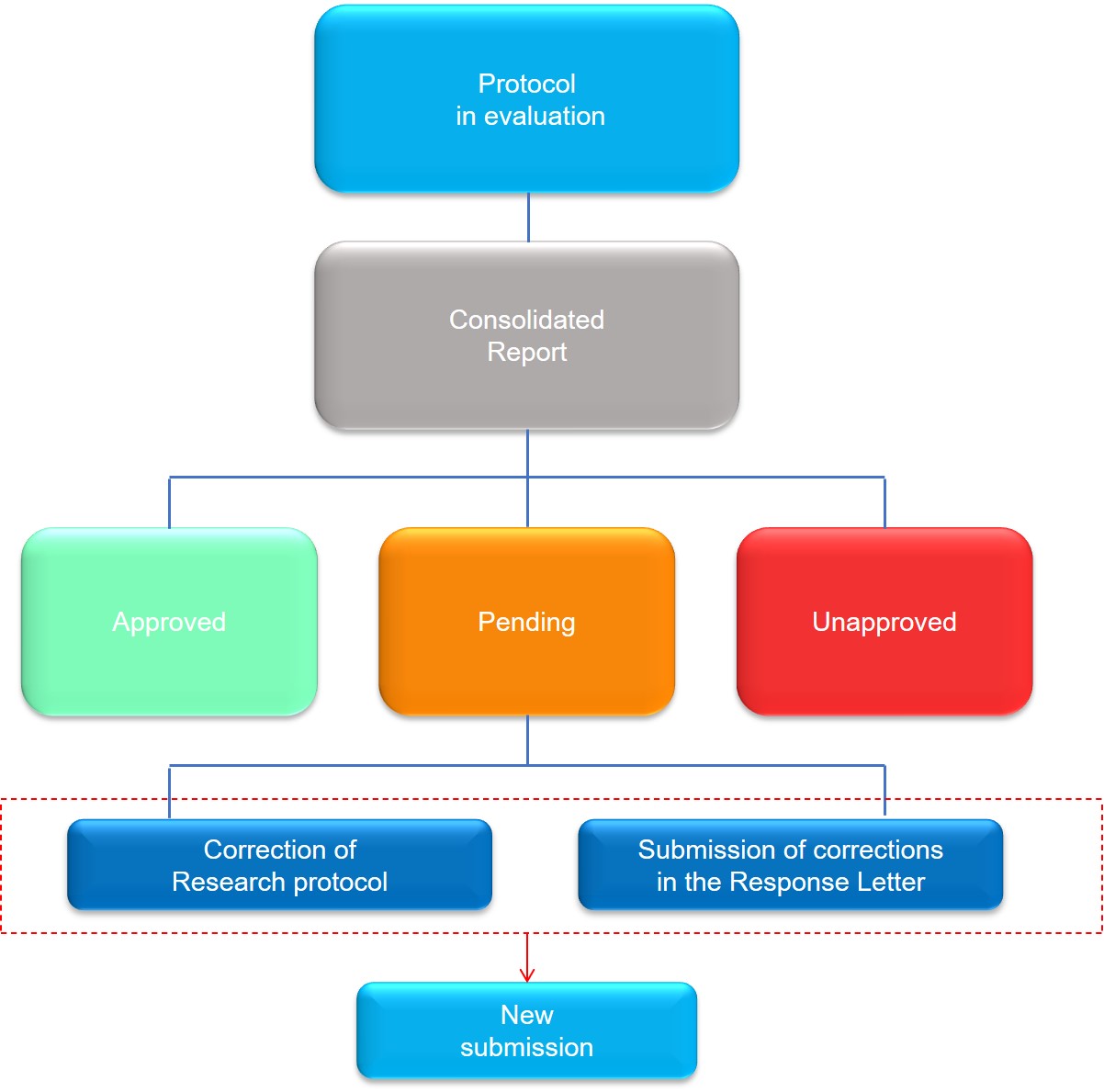
3. “Pending Status Issued by CEP”
“Pending” status means that CEP identified a problem (or some problems) in the research protocol and it needs to be corrected.
The description of pending requests and instructions to correct them will be in the Consolidated Report from CEP, which will be available to the researcher in Plataforma Brasil.
Upon receiving the report, the researcher should perform the correction of all pending requests indicated by CEP-PECEGE in the research protocol documents that require it (cover sheet, research project, consent form, etc.).
In addition to corrections being made directly in the files of documents that presented pending requests and highlighted by red, the researcher should describe all the pending requests in the Consolidated Report and answer them, one by one, in a document called Response Letter.
After performing all corrections in the files and filling in the Response Letter, the researcher should submit a new research protocol, adding all documents again, accompanied by the Response Letter.
The following step by step will instruct on how to do this and, following that, the researcher will find the Response Letter model adopted by CEP-PECEGE.
Step by step to View Pending Project Report
4. “Unapproved” Project Status
The status of “Unapproved” means that CEP identified a very serious problem in the research and it cannot be performed. This decision is rare, but may happen.
To know how to proceed in these cases, follow the step by step below:
Step by step to View Unapproved Project Report
Obs.: When CEP decides to not approve a research project, the responsible may file an appeal to CEP-PECEGE, presenting new facts that have not yet been communicated to CEP and that may lead to a reconsideration of this result. If CEP rejects the appeal, the researcher may ultimately resort to the National Committee of Ethics in Research – Conep, which will evaluate the request of the researcher.
In these cases, contact CEP-PECEGE.
4.1.3.2. Plataforma Brasil – Monitoring the Research Protocol after evaluation
After receiving the consolidated report from CEP- PECEGE stating that their research was “Approved”, the researcher may begin it.
Therefore, after the protocol evaluation process, the stage of monitoring the research development begins.
During the development of research work, some adjustments need to be performed: change of information in the research project (title, place where it will be performed, participants etc.), schedule adjustment etc.
In this case, if the research project suffers any changes or any important event occurs during the performance of the study, the researcher may forward an “Amendment” (appeal to request changes in the research project) or a “Notification” (resource to send relevant reports to CEP about the research project).
Furthermore, CEP-PECEGE will monitor the execution of the work, through partial and final reports.
In summary:
Amendment: is the appeal used to inform CEP of necessary changes in the research protocol after approval
Notification: is the means used to communicate officially to CEP events or important occurrences related to the execution of the research. Partial and final reports, moreover, should be sent to CEP as notifications.
The partial report is a simple report, in which the researcher informs CEP about the progress of the research work.
The final report is the report of the end of the research, in which the researcher communicates CEP of the results of the research and formalizes its end.
1. Alterations in research protocol: “Amendment” submission
After the approval of the research and once the researcher has started the work, he may have to perform minor changes and adequacies (adjust the title, some changes in the schedule etc.). These changes and adjustments are called Amendments and should be sent to the evaluation of CEP-PECEGE.
To understand how to send them, follow the instructions from the step by step below.
Step by step for Amendment Submission
2. Communication with CEP about the research protocol: “Notification” Submission
Whenever the researcher needs to make an important communication about the development of their research, they should do so in the form of a notification. Premature end of the research, submitting the final report of the research, finally, any communication that aims to inform CEP of something related to the research, should be done in the form of notification.
The tutorial below teaches how to submit notifications in Plataforma Brasil.
Step by step for Notification Submission
3. “Partial Report” Submission
The partial report is a simple report, in which the researcher informs CEP about the progress of the research work.
The partial submission deadline is dependent on the research schedule. The recommendation is for the researcher to forward to CEP-PECEGE the partial report when the research development reaches the intermediate stage of the schedule, between the estimate of beginning and ending, when some results have already been produced. Therefore, there is no predetermined deadline for submission and it should be established at the discretion of the researcher themself.
CEP-PECEGE developed a form to facilitate the elaboration of the Partial Report. The researcher should access the form and fill it out. A copy of the document will be sent to the registered email. The researcher should generate a PDF version of the document filled out and submit to CEP-PECEGE through Plataforma Brasil. The process to be followed will be the notification submission process. Follow the step by step bellow.
Step by step for Notification Submission (partial report)
4. “Final Report” Submission
The final report is the report of the end of the research, in which the researcher communicates CEP of the results of the research and formalizes its end.
The deadline for submitting the final report is 30 days after the defense of the final paper.
Submission of the final report is mandatory.
CEP-PECEGE developed a form to facilitate the elaboration of the Final Report. The researcher should access the form and fill it out. A copy of the document will be sent to the registered email. The researcher should generate a PDF version of the document filled out and submit to CEP-PECEGE through Plataforma Brasil. The process to be followed will be the notification submission process. Follow the step by step bellow.
4.2. Organization of research protocol
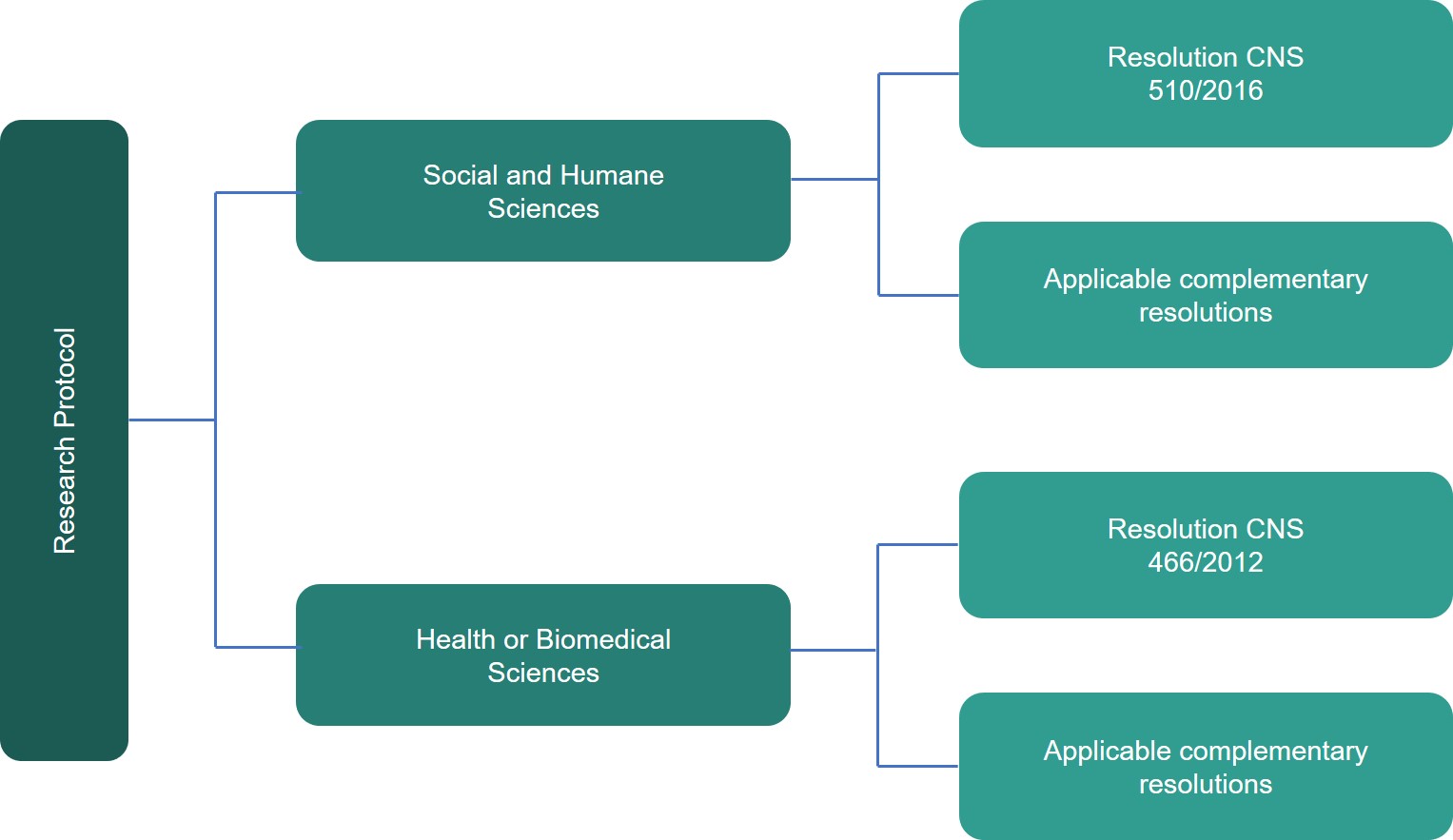
Research protocol is the set of documents that should be sent for the evaluation of CEP-PECEGE. Some of these documents should always be sent. However, others will be required only in certain circumstances, depending on the project that will be performed. What is worth noting is that all applicable documents are mandatory.
The proposed methodology, the profile of the people who will participate in the study, the place in which they will be recruited, the use of secondary data or human biological material, among other characteristics of the research, may require the researcher to submit certain documents, as well as meet the requirements of resolutions applicable to the case. Therefore, the set of mandatory documents will always depend on the characteristics of the research.
The documents presented here will be those that will be used most in research conducted by the academic community of PECEGE.
The documents available on the website of CEP-PECEGE should only be used as models for the works that go through assessment of the Ethics Committee.
Then, if your work did not go through assessment of the Ethics Committee, it is not possible to use the documents available on the website.
Any doubt, seek help from CEP-PECEGE through email<cep@pecege.com>.
The most relevant thing is to understand that documents required by CEP are aimed at ensuring that all involved in the research have the due knowledge of their responsibilities in the course of the study. Therefore, it is necessary to take into account who are the individuals and institutions that, directly or indirectly, will collaborate in the development of the work.
To facilitate identification of documents, they will be classified in the following groups:
- Basic documents
- Declarations and commitments of the researcher
- Declarations and commitments of the co-participant institution
- Documents for research participants.
4.2.1. Basic documents
Basic documents are those that should be presented in any situation for the evaluation of CEP-PECEGE*. They compose the most elementary collection of information about the research and allow CEP to deliberate on the ethical quality of work.
Therefore, it is essential that the researcher is detailed in the preparation of these documents and ensure that they are in accordance with national ethical guidelines.
The documents available on the website of CEP-PECEGE should only be used as models for the works that go through assessment of the Ethics Committee.
Then, if your work did not go through assessment of the Ethics Committee, it is not possible to use the documents available on the website.
The basic documents are:
- Cover sheet
- Detailed research project adapted to the applicable ethical resolutions
- Budget for the research execution (Annex of the Research Project)
- Complete schedule (Annex of the Research Project)
1 – Cover Sheet
The cover sheet is a document generated in Plataforma Brasil, during the registration of the research in the system. It needs to be printed, filled, dated and signed by the responsible researcher, by the responsible person of the proponent institution (in the case of PECEGE students, will be the responsible for PECEGE) and, when appropriate, by the sponsor representative.
Filling of names, document numbers, dates and signatures should be readable and complete.
The title of the research project should be identical to the title of the Research Project and other documents and should be in Portuguese (although the use of one or another concept is accepted in other languages).
ATTENTION: With the exception of cases oriented by CEP – PECEGE, the field “Thematic Areas” should NOT be filled.
In order for the researcher to obtain the signature of the Institutional Responsible in the cover sheet:
- Print the cover sheet that was generated by Plataforma Brasil;
- Sign the Cover Sheet in the section of the Responsible Researcher Signature;
- Scan the already signed Cover Sheet, in good resolution and quality, in .pdf format;
- Send an email to <eticanapesquisa@pecege.com>request the signature of the Responsible for the Proponent Institution, attaching in the email the Cover Sheet already signed by the responsible researcher;
- Wait for the answer wiht the signed document to attach it to Plataforma Brasi and finalize the submission process. Deadline for returning the signed document: 8 working days.
2 – Research Project
The research project is a fundamental part of the protocol. It should be made as the model provided by CEP PECEGE.
Therefore, the research project model made available by PECEGE’s Advisoring team will not have effect if submitted for CEP-PECEGE’s analysis. Therefore, for research that will be sent to CEP’s analysis, it will be necessary to follow the model available on this website (Documents and orientations).
The following contents should be observed in the elaboration of the research project:
- Abstract and Keywords
- Introduction
- Expected results
- Clear project objectives
- Justification
- Material and method/Methodology (Description of methods that directly affect research participants; Description of how and who will obtain the consent form of research participants; Identification of sources for obtaining the research material (database, school files, records etc.)
- Research participants (general characteristics of the population to be studied; Reasons for the use of vulnerable groups)
- Inclusion Criteria, when appropriate
- Exclusion Criteria, when appropriate
- Sample Size
- Research site (description of plans and procedures for recruiting individuals)
- Critical risk analysis (Description and evaluation of possibility and severity of risks)
- Critical analysis of benefits
- Ethical guarantees for research participants
- Description of procedures for monitoring data collection to provide the safety of individuals, including measures to protect confidentiality
- End or suspension of research criteria (Description of measures for the protection or minimization of any possible risk)
- Disclosure of study results
- References
- Annex 1. Instruments that will be used for data collection
- Annex 2. Interview Script
- Annex 3. Schedule with the description of the research stages and, mainly, with the the estimated duration of the research after the approval of CEP.
- Annex 4. Financial budget of the project, which should be appropriate to the expected costs, considering especially the need for compensation of participants
The rule is that these contents are mandatory when applicable to the research design. For example, a research that will be done entirely at distance, without contact with participants and using a digital questionnaire to collect data, will not report the “place where recruitment will be executed”, after all, there will not be a place in a research that will be fully performed on the internet. However, the researcher responsible for this research will still have to present the “description of plans and procedures for recruiting individuals”, since, even using only the internet, they will have to explain how they will invite people to participate in their research.
4.2.2. Declarations and commitments of the researcher
When submitting the research protocol for the evaluation of CEP-PECEGE, three mandatory documents should be included:
- Referral letter
- Commitment statement of the responsible researcher
- Commitment statement of others participating researchers, when applicable.
1 – Referral Letter
In the referral letter, the responsible researcher requests CEP-PECEGE to evaluate the protocol. In addition, the document presents some commitments that the responsible researcher should respect:
- Knowledge of national ethical guidelines.
- Commitment that the research has not yet been initiated.
- Commitment that the research will not involve participation of foreign agents, whether in execution or sponsorship.
- Commitment to communicating CEP-PECEGE whenever an unwanted event and potential risk to participants occurs.
- Commitment to presenting the semi-annual reports and the final report of the research to CEP-PECEGE.
The model for the Referral Letter should be filled, printed, signed by the responsible researcher and included in the Plataforma Brasil with other documents. Only the responsible researcher should sign the document.
In the available model, there is also a checklist of documents to be sent by the researcher. The idea is to allow the researcher to do a control of the documents being submitted.
2- Commitment statement of the responsible researcher
The commintment statement of the responsible researcher should be filled out and signed by the student of the MBA course. In this statement, the researcher assumes the following commitments:
- Comply with national ethical guidelines.
- Use the data obtained only for the purposes described in the project.
- Non-existence of restriction of the disclosure of results or exposure of this restriction.
- Publish research results.
- Report CEP-PECEGE in case of a suspension or early end of the research.
- Report CEP-PECEGE on deviations from the research design and unwanted events.
- Do not start contact with research participants prior to the approval of CEP-PECEGE.
COMMITMENT STATEMENT OF THE RESPONSIBLE RESEARCHER MODEL
3 – Assistant Researcher Statement
The commitment statement of the assistant researcher should be filled out and signed by the advisor and other assistant researchers. In this statement, the assistant researcher assumes the following commitments:
- Comply with national ethical guidelines.
- Use the data obtained only for the purposes described in the project.
- Publish research results.
- Non-existence of restriction of the disclosure of results or exposure of this restriction.
- Report CEP-PECEGE in case of a suspension or early end of the research.
- Report CEP-PECEGE on deviations from the research design and unwanted events.
- Do not start contact with research participants prior to the approval of CEP-PECEGE.
4.2.3. Declarations and commitments of the Co-participant Institution
The Consent Letter is a document that should be filled out with data and signed by the person responsible for authorizing the conduction of the research in co-participating institutions or institutions, that is, institutions in which there will be data collection, recruitment of participants, interview conduction etc.
Whenever research studies depend on access to individuals or data that are associated with or under the custody of a institution or, moreover, depending on access to the interior of their facilities, whether public or private, it will be necessary to request the consent letter to the responsible person.
The role of the Consent Letter is to obtain the authorization of the responsible for a given institution or the safeguard of certain data for the performance of the research.
Therefore, it will be necessary to submit this document when performing the research involves, during recruitment of participants or data collection, the need for access to facilities or databases of a institution, whether public or private. For example, if the work involves data collection in a school, the researcher should request the authorization from the authority responsible for the school (usually the director) to perform the research. This authorization will be formalized in the Consent Letter. The same situation occurs if your research takes place within a company, for example, recruiting participants for the research, or using data from this company.
The document filled out and signed by the responsible of the Institution should be included in Plataforma Brasil.
The rule is that this document is mandatory whenever applicable to the research design. For example, research conducted exclusively with databases available on the internet or interviewing people in public places (in the street, in squares etc.), are exempted from presenting the Consent Letter.
This document ensures that, when performing the research, the researcher was authorized to have access to people or data associated with an institution. When filling in and signing the document, the person responsible for the institution (be a company, school, hospital or academy) states that they have known the objectives of the research and have agreed with its execution in the institution for which they are responsible.
If the research depends on the participation of more than one institution, each of the institutions should sign a Consent Letter and return them to the researcher.
4.2.4. Documents for research participants
The research participant is the person who will be invited to participate in the work and will help you obtain the data and information that the researcher needs; whether answering a questionnaire or participating in an activity, whether providing intimate and private information or, in some cases, even providing biological material, such as blood or cells.
All CEP/Conep System was structured to ensure that, when agreeing to collaborate as a participant of a research, they are treated with respect and dignity and their fundamental rights are respected by the researcher. Therefore, it is necessary for the researcher to have special care when drawing up documents that will be used to invite people to participate in the research work.
Even when the research does not involve direct contact with the participant, such as in the case of medical records, personal information database or even data collection in digital media, it is essential for the researcher to always maintain a posture of respect and responsibility in data handling and data collection provided.
It was based on the concern with these issues that the CEP/Conep System established certain procedures that the researcher should follow when inviting people to participate in their research. The technical name of this procedure is Obtaining Informed Consent of the participant.
The researcher should elaborate a text that will be used in obtaining consent. In general, this text will have the characteristics of an invitation and, as such, need to be written in simple language for easy understanding. As the writing of your research will involve the use of numerous technical concepts, it will be necessary to translate these concepts in the writing of the text that will be used in consent.
This text should always be sent for the analysis of CEP-PECEGE and, therefore, it is one of the mandatory documents of the protocol. (Exceptions, see Waivers from Obtaining Consent).
It is extremely important for the researcher to adjust the process of obtaining informed consent to some situations. These situations have a direct relationship with the profile of the participant that the researcher wishes to recruit for the study.
To help you plan documents that will be used in obtaining the consent of the participants in their research, some profiles will be described below and the researcher should check which profile is the most similar to the profile of participants that the researcher intends to engage in their research.
Participant profile
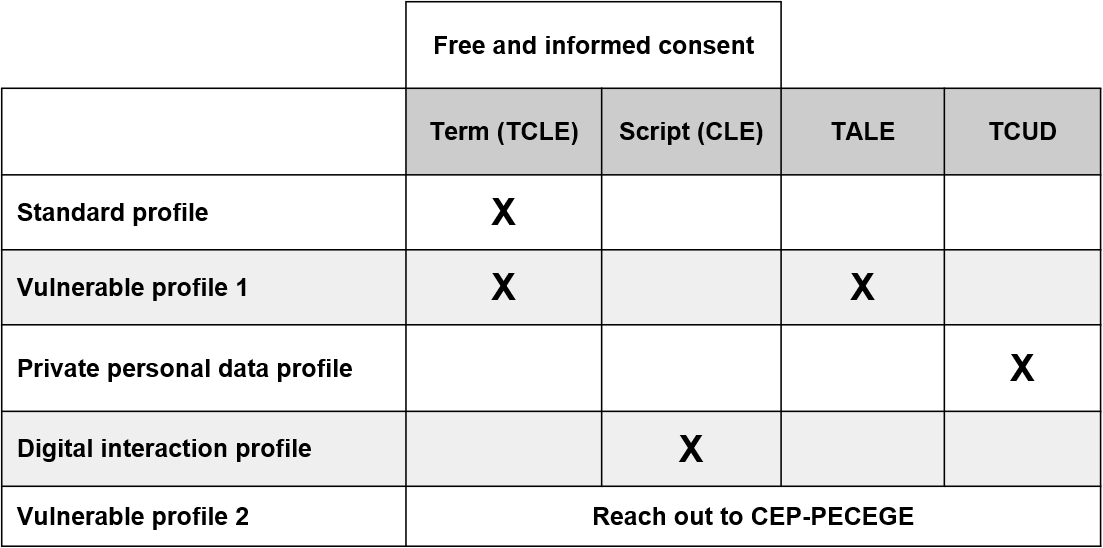
Standard profile – research will only involve the participation:
- of legally capable adults (of age, ≥18, and capable of demanding and fulfilling their rights and performing the typical acts of civil life);
- healthy people (who do not suffer from any clinical condition and are not in any clinical state that may reduce their ability to understand or act according to their will); and
- people free of any constraints, restriction or disciplinary hierarchy that may interfere with their decision to participate or not in the research (for example, prisoners and military members are considered people with reduced autonomy, and therefore they should be avoided involving them in research).
Whenever possible, the researcher should choose this profile of participants.
If participants in the research fall within these characteristics, the researcher should draw up a document called Free and Informed Consent Term (TCLE) that will be delivered to participants who will be invited to participate in the research.
The TCLE should be printed in two copies: one for the researcher and another for the participant. The responsible researcher should sign one of the copies and deliver it to the participant. The participant should initial all pages and return a copy to the researcher.
Based on the profile of research developed in PECEGE, a TCLE model was developed by CEP-PECEGE. This model was developed to meet the requirements of national ethical guidelines, however, it is a generic model. The researcher should fill it with the information and verify whether it was adapted to the design of the research.
FREE AND CLARIFIED CONSENT TERM MODEL – TCLE-PRESENTIAL
Vulnerable profile 1 – research will involve the participation:
- of children or underage teenagers (<18);
- healthy people (who do not suffer from any clinical condition and are not in any clinical state that may reduce their ability to understand or act according to their will); and
- people free of any constraints, restriction or disciplinary hierarchy that may interfere with their decision to participate or not in the research (for example, teenagers admitted at Fundação CASA are considered people with reduced autonomy, and therefore they should be avoided involving them in research).
Children and teenagers are considered a vulnerable group, and therefore, special care involving their participation in research should be adopted. In principle, national ethical guidelines consider that this audience has a limited decision-making capacity, due to their own age and accumulated life experience. Thus, research involving this audience should adopt the following procedure:
-
obtaining the consent of parents or those responsible for the children or teenagers. Elaborating a Free and Informed Consent Term (TCLE) directed to parents or those responsible for the children or teenagers. The TCLE model developed by CEP-PECEGE can be used as a base, however, its content SHOULD BE ADAPTED to the research design.
-
obtaining the consent of the children or teenagers. Registration of this consent should be performed in a document called Free and Informed Assent Term (TALE). TALE should be elaborated according to the ability of comprehension of the participant’s age
TALE does not have a base model. It is of total responsibility of the researcher to choose the best way to elaborate the content of this document that may include playful resources or illustrations to facilitate the understanding of the child or teenager. The important thing is to ensure that, under the limit of their capacities, these participants may manifest their desire to participate in the research.
FREE AND INFORMED ASSENT TERM – TALE MODEL
TALE should be printed in two copies: one for the researcher and another for the participant. The researcher should sign one of the copies and deliver it to the responsible for the child or teenager. The responsible for the participant should initial all pages and return a copy to the researcher.
FREE AND INFORMED CONSENT TERM MODEL – TCLE – PRESENTIAL – PARENTS-LEGAL GUARDIAN
Vulnerable profile 2 – research will involve the participation:
- of legal or clinically incapable person;
- of person in a state of temporary disability;
- of a member of indigenous community;
- of person in condition of embarrassment, restriction of freedom or subject to rigid disciplinary hierarchy.
If your research will depend on the inclusion of people with some of the characteristics above, it is recommended that the researcher seek CEP-PECEGE to receive the correct instructions and clarify the necessary doubts before elaborating the protocol.
Digital interaction profile
Data collection and interaction with the participant (direct or indirect) may be totally digital, especially through digital forms or surveys.
If the research maintains only digital interaction with participants, the researcher should develop a script for Informed Consent (CLE), which will be presented to people who will participate in their research.
After the approval of CEP-PECEGE, this text should be included in the digital platform that will be used to perform the research.
The researcher should make it available before the data collection instrument.
Data collection instrument can only be accessed after reading and manifesting agreement between the participant with the contents of the CLE. This process will serve as a participant authentication, which will express their consent.
Once the participant has expressed their agreement, the researcher should provide a version of the CLE for download, so that the participant may have a copy of the document.
The CLE script may be built from the CLE model developed by CEP-PECEGE. However, the researcher should fill it with the information and verify whether it was adapted to the design of the research.
INFORMED CONSENT MODEL – CLE – MODEL
Private personal data profile
The research will involve consulting private personal data, kept under the supervision of a specific institution, and there will be no interaction with data owners (participants).
In this case, the researcher should request the authorization of the institution responsible for the safeguarding of private personal data to access them. The researcher needs to use the document called Data Use Commitment Term (TCUD).
TCUD is the document in which the responsible researcher and their team commit to the confidentiality and privacy of research data, as well as the commitment to be used only for the study in question. It is a substitute for the Free and Informed Consent Term (TCLE) and, again, should be used when data that will be accessed are proprietary data maintained on the safeguard of a given institution.
The most common condition that will require the use of TCUD is the research that will use medical records. The records are owned by the patient, but the hospital safeguards the document and its information. Therefore, whenever a research intends to use medical records, it is necessary to request the TCLE of the medical records’ owners (patients). If it’s impossible to locate them, it will be necessary to replace the TCLE with the TCUD.
Based on the profile of research developed in PECEGE, a TCUD model was developed by CEP-PECEGE. This model was developed to meet the requirements of national ethical guidelines, however, it is a generic model. The researcher should fill it with the information and verify whether it was adapted to the design of the research.
Synthesis Framework
Based on the profile of participants who will be recruited in the research, here are indicated the documents for the research participant that the researcher should elaborate and refer to the evaluation of CEP-PECEGE.
Waiver of Consent Obtention
There are cases where there is no waiver from the TCLE registry:
when there are risks to privacy and confidentiality of the participant: according to CNS 466/212 and CNS 510/2016: “In cases where it is unfeasible to obtain the Informed Consent Term or that this obtention means substantial risks to privacy and confidentiality of data from the participant or of trust links between researcher and researched, the exemption of the TCLE should be requested justifiably by the researcher responsible for the CEP/CONEP System, for assessment, without prejudice to the subsequent clarification process”. For example, research on illicit practices.
when there are risks for establishing trust relationship between researcher and researched, according to CNS 510/2016: “[case in which] registration means substantial risks to privacy and confidentiality of data from the participant or to the links of trust between researcher and researched, the waiver should be justified by the researcher responsible for the CEP/CONEP system”. Example: research on sexuality with teenagers.
research involving the use of medical records, but when it’s not possible to access participants to obtain permission for consultation (participants who are not in service or cannot be contacted, with due justification): for these cases, commitment of the researcher to the institution of records using TCUD (Data Use Commitment Term);
research that does not allow the identification of the participant or their tracking, in which data of participants are strictly anonymous do not require the registration of the TCLE in written form, but do not exempt from the clarification process, which should be provided on the first page of the form created for data collection. Example: urns distributed in a school/hospital for opinion research.
Even if there is no registration of consent and/or consent registry, it is necessary to have the consent process and/or assent process, as provided in CNS resolution 510/216: “If there is no registration of consent and assent, the researcher should submit a document to the participant that contains the information provided for free and informed consent regarding the research”.